Waldenström Macroglobulinemia (WM) is an incurable lymphoma. Despite the advances in molecular characterisation of WM and the development of novel drugs such as Bruton tyrosine kinase inhibitors, rates of deep response with complete remission remain very low at ~ 30%. Therefore, newer and more effective treatments are needed for WM. Novel drug development is hampered by high costs and failure rates, and long development times. These limitations and the unmet need for anti-cancer therapies have created a growing interest in repurposing drugs that are already approved for other indications to manage cancer. To identify safe and effective therapeutic options for the treatment of WM, we performed an unbiased high-throughput in vitro drug screening at the Australian National University Centre for Therapeutic Discovery. We used a cell line derived from a patient with WM called MWCL-1 to discover drug candidates with cytotoxicity potential. We treated these cells with 2,393 compounds approved by the Food and Drug Administrator (FDA) using five different concentrations and measured the cell viability using Cell Titre Glo assay which quantifies the amount of ATP released from the viable cells. In addition, we performed live cell imaging using Incucyte to confirm the cytotoxic potential of lead compounds. Furthermore, immunoblotting was used to identify the programmed cell death pathway induced by the lead compounds.
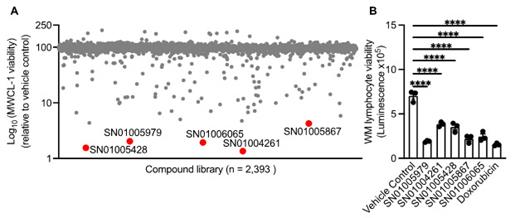
Our high-throughput screen of 2,393 compounds from the FDA library identified 33 drug candidates which substantially reduced the viability of MWCL-1 cells as compared to vehicle control (dimethyl sulfoxide). This screen yielded a hit rate of 1.3%, based on the cut-off score of IC 50 2 μM or less. Of these hits, we identified five highly potent compounds with an IC 50 of less than 0.1 μM ( Fig. 1A). We confirmed the cytotoxicity potential of these lead compounds and found that all five compounds reduced the viability of MWCL-1 cells as early as 12 hours after treatment, with the highest reduction in viability observed at 72 hours. Furthermore, these compounds substantially reduced the viability of another WM cell line called BCWM.1 which morphologically and phenotypically resembles lymphoplasmacytic cells of WM. Importantly, we observed that these compounds reduced the viability of bone marrow-derived primary lymphocytes from a patient with WM as compared to vehicle control ( Fig. 1B). The compound SN01005979 was most effective and exerted a similar cytotoxic potential as standard treatment Doxorubicin ( Fig. 1B). To characterize the mechanisms of action by which these lead compounds kill MWCL-1 and BCWM.1 cells, we performed immunoblotting and tested the activation of programmed cell death pathways including caspase-1, gasdermin (GSDM)-D, GSDME (pyroptosis), caspase-8 (extrinsic apoptosis), caspase-9 (intrinsic apoptosis), caspase-3, -6, and -7 (executioners of apoptosis), and phosphorylation of MLKL (necroptosis). We found that these compounds selectively induce caspase-3-dependent cleavage of GSDME.
In summary, using a high throughput screen, we identified novel drug candidates with potent cytotoxic activity against WM. Our identification of key molecules targeted by these drug compounds could pave the way for the treatment of tumours expressing caspase-3 and GSDME. Moreover, our results offer new insights into the mechanism of certain FDA-approved drugs and could help in the development of safer and more effective cancer chemotherapy against WM and potentially other cancers. Future studies are required to test the tumour-killing potential of these drugs in vivo using mouse and humanised mouse models of WM.
Disclosures
Talaulikar:Amgen: Honoraria; Beigene: Honoraria; Takeda: Honoraria; CSL: Honoraria, Speakers Bureau; EUSA: Honoraria; Antengene: Honoraria; Roche: Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria, Research Funding, Speakers Bureau.